Evaluation of Transdermal Formulations: A Technical Note
Mudasir Mohamad, Roheena Jan
Pages: 37-40

Design of dissolution media for in-vitro bioequivalence testing of Lamivudine
Nagiat T Hwisa, Shanta Kumari Adiki, Prakash Katakam, Babu Rao Chandu
DOI: 10.7324/JAPS.2013.3617Pages: 106-110

Development and Validation of a Stability Indicating Spectrofluorimetric Method for the Determination of Lanzoprazole via its Degradation Product
Ghaleb Oriquat, Afaf Osman, Mohammad Abdul-Azim and Sawsan Abuhamdah
DOI: 10.7324/JAPS.2014.40410Pages: 057-061

DoE Approach: A Stability Indicating RP-HPLC Method for Simultaneous Estimation of Methylparaben, Mometasone furoate and Eberconazole nitrate in Topical Formulations
Prakashkumar B. Modi, Nehal J. Shah
DOI: 10.7324/JAPS.2014.41204Pages: 020-025

Stability Indicating RP-HPLC Assay Method for Estimation of Dronedarone Hydrochloride in Tablet
Atul T Hemke, Shivshanker Kukade, Rajesh T Lohiya, Krishna R Gupta
DOI: 10.7324/JAPS.2015.50516Pages: 083-088

A novel stability indicating HPLC-method for simultaneous determination of atenolol and nifedipine in presence of atenolol pharmacopeoial impurities
Hisham Hashem, Ibrahim Adel Ehab, Elhenawee Magda
DOI: 10.7324/JAPS.2015.50804Pages: 017-025

Validated, Ultra High Efficiency RP-HPLC and Stability Indicating Method for Determination of Tranylcypromines Sulphate in Bulk and in Tablet Dosage Forms
Gamal H. Ragab, Hanaa M. Saleh, Magda M. EL-Henawee, Omnia F. Elsayed
DOI: 10.7324/JAPS.2016.60209Pages: 064-071

Development and Validation of a Stability Indicating HPLC Method for the Simultaneous Analysis of Esomeprazole and Itopride in Bulk and In Capsules
M. Nageswara Rao, K. B. M. Krishna, B. Hari Babu
DOI: 10.7324/JAPS.2016.60210Pages: 072-080

Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Sumatriptan in Bulk and Pharmaceutical Dosage Form
M. Srinidhi, Md. Mushabbar Basha, V. Raj Kumar, J. Rajendra Kumar
DOI: 10.7324/JAPS.2016.60604Pages: 020-025

Development and validation of a stability indicating HPLC-diode array-fluorescence method for the determination of meclofenoxate hydrochloride and p-chlorophenoxyacetic acid
Marwa Said Moneeb, Feda Elgammal, Suzy Mohamed Sabry
DOI: 10.7324/JAPS.2016.60701Pages: 001-011

Development of Validated Stability Indicating RP-HPLC-PDA Method for Camptothecin Analysis
Buchi N. Nalluri, Saisrianusha Valluru, Chandrapriyanka Bonthu
DOI: 10.7324/JAPS.2016.60921Pages: 140-146

Stability Indicating HPLC Method for the Simultaneous Quantification of Aspirin and Pravastatin in bulk and Tablets: Method Development and Validation
Ravi Varma Athota, Shanmukha Kumar Jagarlapudi, Mutta Reddy Singampalli
DOI: 10.7324/JAPS.2017.70308Pages: 048-056

A Sensitive, Stability indicating UPLC method for the identification and characterization of forced degradation products for Drometrizole Trisiloxane through MSn studies
M. Ajay Babu, G. V. Krishna Mohan, J. Satish, Pradipbhai D. Kalariya, CH. Krishnam Raju, Sharad D. Mankumare
DOI: 10.7324/JAPS.2018.8609Pages: 065-074

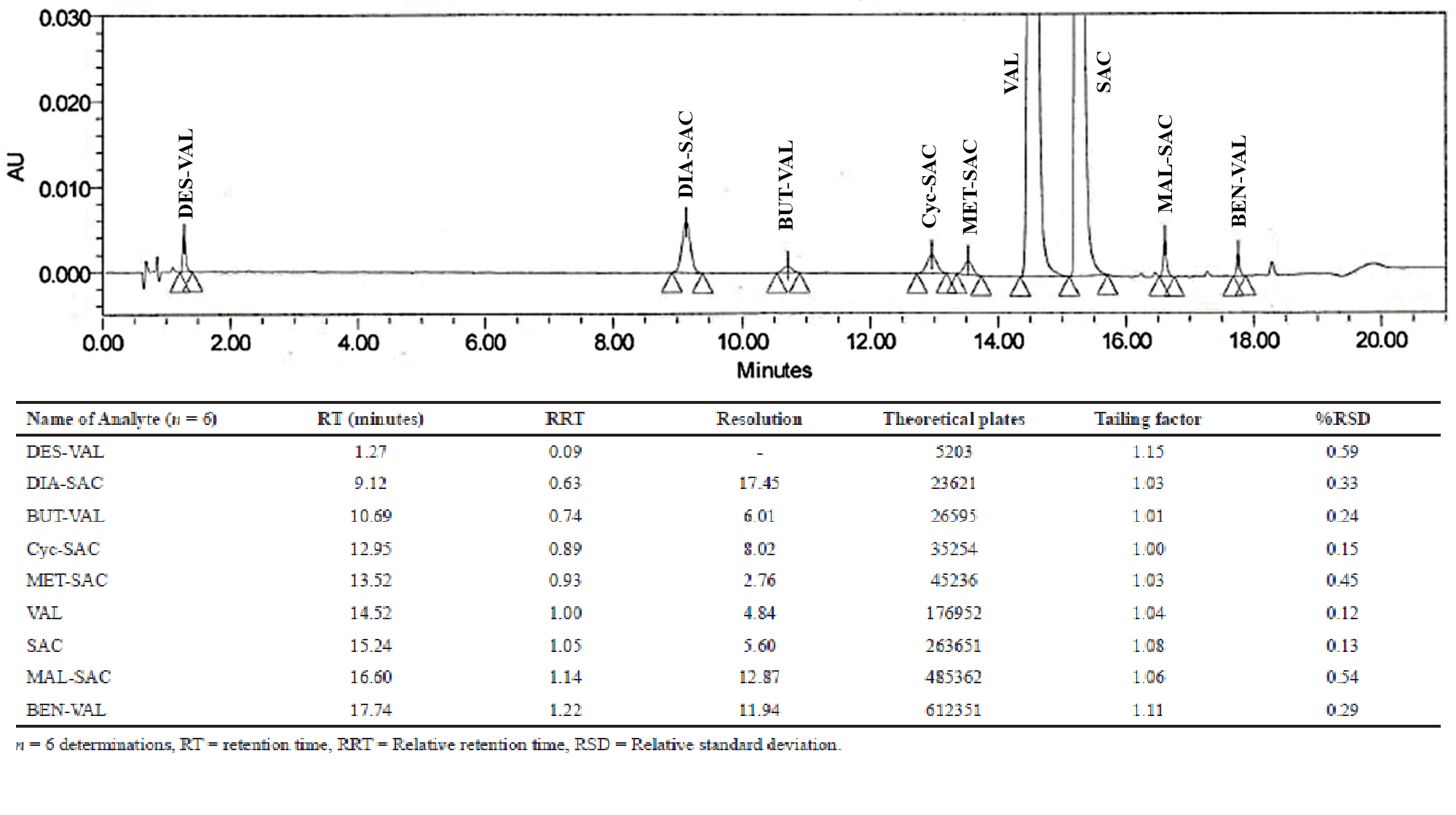
Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107
A stability-indicating reverse phase-HPLC method development and validation for newly approved drug, Belzutifan in bulk and pharmaceutical dosage forms
Dumpala Sravya, Banoth Ramya Kuber
DOI: 10.7324/JAPS.2023.114351Pages: 233-240